
Danielle Barth
University of Lorraine, France
Title: Supercritical carbon dioxide extraction processes development
Biography
Biography: Danielle Barth
Abstract
In supercritical CO2 extraction process, there are two essential steps: the extraction step in the extractor where the SC-CO2 allows the solvent removal from product structure and the separation step which consists of the separation of CO2-solvents in a cascade of cyclone separators downstream the extractor. Cyclone separators are separation devices that use the centrifugal and gravity forces to remove liquid phase from flue gases. Two supercritical extraction processes are studied here: organogels supercritical drying for aerogels production and supercritical extraction of polar compounds from natural products. Concerning the first process, the organogel is prepared by an aminoacid-type organogelator with aromatic solvents such as tetralin or toluene. The experimental results showed a good solvent recovery rate in the case of tetralin, exceeding 90% but an unsatisfactory separation for toluene with a yield below 65%. In order to understand the experimental results, a thermodynamic study and a hydrodynamic study (CFD) of the mixture separation in the cyclones are carried out. Supercritical extraction of polar compounds from natural products using a CO2 + aqueous ethanol mixture as solvent requires a reliable knowledge of vapor-liquid equilibria of the carbon dioxide + ethanol + water system in order to size and optimize the extraction process. The purpose of this study is to select an appropriate thermodynamic model among the ones available in commercial process simulators for representing the phase behaviour of the system of interest. This study highlighted that the optimal thermodynamic models for the application of interest have to be chosen among the VTPR, PSRK and MHV2-UNIFAC EoS. Once identified a suitable thermodynamic model for the CO2 + ethanol + water ternary system, it has been possible then to simulate the extraction process of polar compounds from natural products and to discuss how water influences the process efficiency.
Image
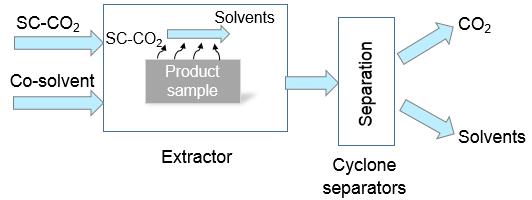
Figure 1: Simplified scheme of supercritical extraction
Publications
- Bensebia O, Bensebia B, Allia K, Barth D (2016) Supercritical CO2 extraction of triterpenes from rosemary leaves: Kinetics and modelling. Separation Science and Technology 51,13: 2174-2182
- Lazrag M, Mejia-Mendez DL, Lemaitre C, Emmanuel Stafford PH, Hreiz R, Privat R, Hannachi A, Barth D (2016) Thermodynamic and hydrodynamic study of a gas-liquid flow in a cyclone separator downstream supercritical drying. J Supercrit Fluids 118:27–38
- Jamart-Grégoire B, Son S, Allix F, Felix V, Barth D, Pickaert G, Degiovanni D (2016) Monolithic organic aerogels derived from single amino-acid based supramolecular gels: physical and thermal properties. RSC Adv 6:102198–102205
- Lazrag M, Steiner E, Lemaitre C, Mutelet F, Privat R, Rode S, Hannachi A, Barth D (2017) Experimental and thermodynamic comparison of the separation of CO2/toluene and CO2/tetralin mixtures in the process of organogel supercritical drying for aerogels production, J Sol-Gel Sci Technol
- Ravetti Duran R, Escudero Falsetti P, Muhr L, Privat R, Barth D, Phase equilibrium study of the ternary system CO2+H2O+Ethanol at elevated pressure for the supercritical extraction of polar compounds from natural products, 16th European Meeting on Supercritical Fluids 25-28 April 2017 Lisbon

