Day 2 :
Keynote Forum
Aleksandra Sander
University of Zagreb, Croatia
Keynote: The role of green solvents in purification of fuels
Time : 09:30-10:00

Biography:
Aleksandra Sander received the MEng and PhD degrees in chemical engineering in 1999 and 2003, respectively, from the Faculty of Chemical Engineering and Technology (FCET) University of Zagreb, Croatia. She is working at the Department of Mechanical and Thermal Process Engineering at FCET since 1994 and she was elected full professor in 2011. She published around 50 papers (journal articles and conference papers). She took part in 35 international and national conferences. She participated in the execution of several national science projects supported by the Croatian Ministry of Science and Technology and the Croatian Science Foundation. She was leader of the research project supported by Croatian Academy of Sciences and Arts. Her research interest includes thermal separation processes like drying, crystallization and liquid-liquid extraction.
Abstract:
During the past decade, a growing trend of global energy consumption can be observed. This is particularly true for the transportation sector, which mainly relies on the fossil fuels. As a result, increased emissions of combustion products (SOx, NOx, CO2) that adversely influence the environment and are responsible for the global warming and climate changes can be detected. One way for reduction of harmful emissions is to produce fuel with ultra-low sulfur and nitrogen content. Commercial processes for desulfurization and denitrification require high temperatures and pressures, as well as large amounts of hydrogen, making purification step an expensive and relatively environmentally unfriendly process. To reduce the emission of the greenhouse gasses, fossil fuels should be replaced with renewable, sustainable alternative fuels that must be ecologically and technologically acceptable, economically competitive and easily available. Biodiesel is one of such fuels. Requirements for the development of new technologies for the production of biodiesel from the so-called advanced raw materials – waste cooking oil and waste animal fats as well as from the microalgae come out of the increased world demand for energy. Among alternative methods for lowering sulfur and nitrogen content of FCC gasoline and diesel fuel, as well as for pretreatment of raw materials for biodiesel production and purification of raw biodiesel, liquid-liquid extraction with ionic liquids and deep eutectic solvents can be highlighted. The selection of the appropriate solvent involves the experimental determination of liquid-liquid equilibria and derivation of a mathematical model, which would allow for the evaluation of the extraction efficiency. Short review of the application of green solvents in the processes of separation of impurities from fossil fuels and biodiesel will be presented.
- Advancements in Sample Preparation Techniques | Separation Processes in Chemical Engineering | Applications of Separation Techniques
Location: Paris, France
Session Introduction
David Chiche
IFP Energies Nouvelles, France
Title: COSWEETTM: A new process to reach very high COS specification on natural gas treatment combined with selective H2S removal

Biography:
David Chiche did PhD in materials science from Pierre et Marie Curie University (Paris, France). He has been working in the Separation Department at IFP Energies Nouvelles for 10 years. His expertise was developed in the field of Synthesis Gas and Natural Gas Purification Technologies, especially in relation with the Development of Metal Oxides Adsorbents, Catalysts and related processes.
Abstract:
Natural gases are commonly polluted with many contaminants such as sulfur compounds and CO2. Throughout the oil and gas treatment chain, various steps intended to separate most of the undesired compounds from the profitable part of the natural gas. Natural gas desulfurization is usually performed in generic treatment processes and in order to remove H2S and/or CO2 to meet the export gas specifications. However, commercial gas specifications are not restricted to H2S and CO2. New specifications have been imposed for many years to also remove most of other sulfur compounds from the natural gas. One of these, Carbonyl Sulfide (COS) is usually present in sour natural gases containing both H2S and CO2, in quantities which may exceed 200 vol. ppm. COSWEETTM process, developed for the treatment of COS containing natural gases, is based on a combination of deacidification through any alkanolamine solution with a COS hydrolysis section over on a metal oxide based catalyst. Nearly complete COS conversion is reached, even at a relatively low operating temperature. Coupled with a classical sweetening unit, in which an adapted alkanolamine solvent is used in order to optimize the removal of H2S, CO2, as well as the H2S/CO2 selectivity, the high catalyst activity and the original integration of the scheme secure the COS removal at minimum extra cost. Benefits of capital and operating expenditures of the plant result both from the reduction of the absorption column and solvent flow rate and from the quality of the acid gas, which has positive consequences on the design of the sulfur recovery facilities units, including Claus unit.The approach set for the process development and the results obtained on COS conversion will be presented, as well as the model and simulation tool, and a case study showing the advantages of coupling COSWEETTM to amine-based solvent.
Image
 COSWEETTM Process scheme
COSWEETTM Process scheme
Publications
- J. Magné-Drisch, J. Gazarian, S. Gonnard, J.M. Schweitzer, D. Chiche, G. Laborie, G. Perdu, COSWEETTM: A New Process to Reach Very High COS Specification on Natural Gas Treatment Combined with Selective H2S Removal, Oil Gas Sci Technol, 2016, 71, 40.
- D. Chiche, J.M. Schweitzer, Investigation of competitive COS and HCN hydrolysis reactions upon an industrial catalyst: Langmuir-Hinshelwood kinetics modeling, Appl Catal B-Environ, 2017, 205, 189-200.
- D. Chiche, C. Diverchy, A.-C. Lucquin, F. Porcheron, F. Defoort, Synthesis Gas Purification, Oil Gas Sci Technol, 2013, 68(4), 707-723.
- V. Girard, D. Chiche, A. Baudot, D. Bazer-Bachi, I. Clémençon, F. Moreau, C. Geantet, Innovative low temperature regenerable zinc based mixed oxide sorbents for synthesis gas desulfurization, Fuel, 2015, 140, 453-461.
- L. Neveux, D. Chiche, J. Perez-Pellitero, L. Favergeon, A.S. Gay, M. Pijolat, New insight into the ZnO sulfidation reaction: mechanism and kinetics modeling of the ZnS outward growth, Phys Chem Chem Phys, 2013, 15, 1532-1545.
Danielle Barth
University of Lorraine, France
Title: Supercritical carbon dioxide extraction processes development

Biography:
Danielle Barth contributes to develop Supercritical carbon dioxide processes (continuous and batch extraction, prep-scale supercritical fluid chromatography, drying, dying, VOC desorption, Chemical reactions (like Staudinger Aza-Wittig), Enzymatic reactions (with lipase) at pilot-scale and laboratory-scale since thirty years.
Abstract:
In supercritical CO2 extraction process, there are two essential steps: the extraction step in the extractor where the SC-CO2 allows the solvent removal from product structure and the separation step which consists of the separation of CO2-solvents in a cascade of cyclone separators downstream the extractor. Cyclone separators are separation devices that use the centrifugal and gravity forces to remove liquid phase from flue gases. Two supercritical extraction processes are studied here: organogels supercritical drying for aerogels production and supercritical extraction of polar compounds from natural products. Concerning the first process, the organogel is prepared by an aminoacid-type organogelator with aromatic solvents such as tetralin or toluene. The experimental results showed a good solvent recovery rate in the case of tetralin, exceeding 90% but an unsatisfactory separation for toluene with a yield below 65%. In order to understand the experimental results, a thermodynamic study and a hydrodynamic study (CFD) of the mixture separation in the cyclones are carried out. Supercritical extraction of polar compounds from natural products using a CO2 + aqueous ethanol mixture as solvent requires a reliable knowledge of vapor-liquid equilibria of the carbon dioxide + ethanol + water system in order to size and optimize the extraction process. The purpose of this study is to select an appropriate thermodynamic model among the ones available in commercial process simulators for representing the phase behaviour of the system of interest. This study highlighted that the optimal thermodynamic models for the application of interest have to be chosen among the VTPR, PSRK and MHV2-UNIFAC EoS. Once identified a suitable thermodynamic model for the CO2 + ethanol + water ternary system, it has been possible then to simulate the extraction process of polar compounds from natural products and to discuss how water influences the process efficiency.
Image
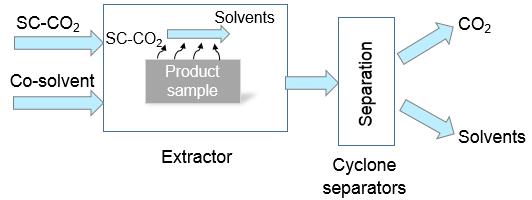
Figure 1: Simplified scheme of supercritical extraction
Publications
- Bensebia O, Bensebia B, Allia K, Barth D (2016) Supercritical CO2 extraction of triterpenes from rosemary leaves: Kinetics and modelling. Separation Science and Technology 51,13: 2174-2182
- Lazrag M, Mejia-Mendez DL, Lemaitre C, Emmanuel Stafford PH, Hreiz R, Privat R, Hannachi A, Barth D (2016) Thermodynamic and hydrodynamic study of a gas-liquid flow in a cyclone separator downstream supercritical drying. J Supercrit Fluids 118:27–38
- Jamart-Grégoire B, Son S, Allix F, Felix V, Barth D, Pickaert G, Degiovanni D (2016) Monolithic organic aerogels derived from single amino-acid based supramolecular gels: physical and thermal properties. RSC Adv 6:102198–102205
- Lazrag M, Steiner E, Lemaitre C, Mutelet F, Privat R, Rode S, Hannachi A, Barth D (2017) Experimental and thermodynamic comparison of the separation of CO2/toluene and CO2/tetralin mixtures in the process of organogel supercritical drying for aerogels production, J Sol-Gel Sci Technol
- Ravetti Duran R, Escudero Falsetti P, Muhr L, Privat R, Barth D, Phase equilibrium study of the ternary system CO2+H2O+Ethanol at elevated pressure for the supercritical extraction of polar compounds from natural products, 16th European Meeting on Supercritical Fluids 25-28 April 2017 Lisbon

Biography:
Margaret C Enedoh has completed her PhD from University of Abuja Nigeria. She is a lecturer in Imo State University Owerri, Nigeria. She is a member of National Science Teachers of America and America Chemical Society and back home belongs to Chemical Society of Nigeria and is currently the coordinator of Female Chemists in Imo state Nigeria. She is a staunch member of Science Teachers Association of Nigeria (STAN) and the national chairperson of the chemistry panel of STAN. She organizes and coordinates national chemistry workshops yearly. She is highly interested in scientific research works.
Abstract:
Extraction of oil from palm kernel nut was carried out using two local methods practiced in the Eastern part of Nigeria in Africa – Mechanical extraction and Solvent extraction. The palm kernel nuts were cracked and the kernel – shell separation done by density – clay – bath. Using mechanical extraction, the nuts were cleaned by pre-treatment, followed by size reduction through grinding, then, flaking and steam conditioning. The resultant meals were passed through mechanical stirring agitator and then subjected to screw-press which forced the meals through the barrels in series till oil drained out through perforated bars while the solid-cake discharged through the annular orifice. In solvent extraction the kernel nuts after pre-treatment were dried, ground to paste, mixed with little water and heated to release the oil which surged out from the paste and remain on top and was scooped out. The oils obtained were characterized and the physicochemical properties were same as follows: saponification valule (SV) 322.575, iodine value 25.33mg/kg, acid value (AV) 7.4824 and free fatty acid value (FFAV) 3.76. These values proved that the palm kernel oil extracted locally as described has similar characteristics with those obtained through advanced mechanism and so, proves the two extraction methods suitable.
Publications
1.Cnidi Edbert Duru, Ijeoma Akunna Duru, Francis Chizoruo Ibe and Margaret Chinyelu Enedog (2017) Profiling of Zn2+ ion sorption in modeled aqueous solution by different parts of maize biomass. IOSR Journal of Applied Chemistry (IOSR-JAC) 10,70-75
2. F.C Ibe, B.O Ibe, C.B.C Ikpa, M.C. Enedoh, (2016) Remediation of mild crude oil polluted fresh water wet land with organic and ionorganic fertilizer. International Letters of Natural Sciences. 54, 75-85
3. M.C.Enedoh (2915) Complexes of 2-amino-4-thiazoleacetic acid hydrazide (ATAH), Salicyladehyde-2-amino-4-thiazoleacetic acid hydrazone(ATASH) and Acetone-2-amino-4-thiazoleacetic acid hydrazone(ATAAH) eacg with copper(II)sulphate. International Journal of Scientific & Engineering Research. 6, 110-116.
4 M.C. Enedoh and J.N Nwabueze(2011) Complexes of 4-cyanobenzaldehydecyclohexylacetic acid hydrazone with some M(II) sulphates and acetates (M=Ni,Cu,Co,Zn) Zuma Journal of Pure & Applied Science. 9, 1-7
5 M.C. Enedoh, F.C. Ibe and P.N. Ebosie(2016) Complexes of 2-amino-4-thiazoleacetic acid hydrazone, salicylaldehyde-2-amino-4-thiazoleacetic acid hydrazone and acetone-2-amino-4-thiazoleacetic acid hydrazone each with nickel(II) sulphate. African Journal of Education, science andTechnology(AJEST) 3,143-147.
Maria Vilma Faustorilla
University of South Australia, Australia
Title: Fractionation of total petroleum hydrocarbons in soil by SPE-GC for toxicity studies to Eisenia fetida

Biography:
Maria Vilma Faustorilla obtained her Bachelor and Master of Science degrees in Chemistry from the University of the Philippines. She has accumulated many years of experience in R&D, QC, QA, Clinical and manufacturing environments in the pharmaceutical industry, having worked within various highly regulated environments that are NATA, TGA or FDA accredited. She is currently a PhD student of the University of South Australia in the environmental strand of the Future Industries Institute, which develop solutions to complex contamination problems
Abstract:
As petroleum is a complex mixture of hydrocarbons, the ecotoxicity of petroleum-contaminated soils has been based mostly on the total petroleum hydrocarbon (TPH) load. However, a requirement of environmental fate and risk analysis is the separation of TPH into its aliphatic and aromatic components appropriately due to the toxicity differences between the fractions. This study developed a fractionation method for TPH in soil using solid phase extraction (SPE) technique followed by analysis utilizing gas chromatography (GC) coupled to mass selective detector and flame ionization detector systems. Validation results suggested that method performance are related to molecular weight, molecular structure and soil organic matter content; thus, providing important information on the behaviour of the individual hydrocarbons such as adsorption into soil which is significant in determining environmental fate and weathering of hydrocarbons. Three soil samples contaminated with aliphatic and aromatic components were collected to determine earthworm chronic toxicity. Lethality and sub-lethal effects on days 7, 14, 21 and 28 were investigated on Eisenia fetida. The toxicity studies showed that aromatic fractions exerted increased lethal effects upon concentration on earthworms occurring at lower concentrations as compared to the aliphatic fractions, which only showed adverse effects at higher concentrations. This work emphasized the need to determine the fractions of TPH in a contaminated soil, instead of using TPH as a single hydrocarbon index, to have a more accurate assessment towards ecological hazards and impact.
Rouhollah Ahmadi
Iran University of Science & Technology, Iran
Title: Clean desalination using reverse osmosis system providing pressure by energy harvesting from ocean waves

Biography:
Rouhollah Ahmadi is an Assistant Professor of Mechanical Engineering at the Iran University of Science and Technology from 2013. His teaching and research are focused on Thermal- hydraulic, Optimization, and Thermal energy storage systems as it applies to energy systems. His work goals are to (1) educate/develop mechanical engineers/workforce, and (2) help move advanced energy systems from arts to science-based technologies that will help innovate optimize the system. These include energy efficiency of energy systems, latent thermal energy storage system, exergo-economic analysis, renewable energy, desalination and heat pipes.
Abstract:
Statement of the Problem: The size of dirt particles contained in seawater varies widely. A very special filtration solution is required to remove particles from 10 mm at one end of the spectrum down to 50-micron particles at the other. Reverse osmosis (RO) water system is one of the promising methods that are employed in filtration of fine particles during desalination of seawater. However, this method requires huge energy to provide high pressure in the system. On the other hand, the growing of energy demand besides of the limitations of fossil fuels and hazardous impact of the environmental pollution caused by the consumption of fossil fuels makes renewable energies as attractive energy recourses. In this study, a desalination system using RO technique is introduced that its pressure is provided by energy harvesting from ocean waves. One of the sources of renewable energy, besides each desalination system, is sea waves. This paper aims to design and analyze the RO system connected to the sea waves energy harvester system. This system can be useful for the remote islands where they have a problem to access to potable water and common energy resources. To design a system for extracting energy from sea waves CFD model of special floating geometry was investigated. This floater can harvest of buoyancy energy as well as the inertial energy of wave impact. The behavior of floater is simulated by passing sea waves to find out the amount of energy harvesting. A hydraulic system connected to the floater to convert reciprocating motion to pressure in the accumulator. This pressure can be transfer to the RO system for refining filtration of sea water.
Image

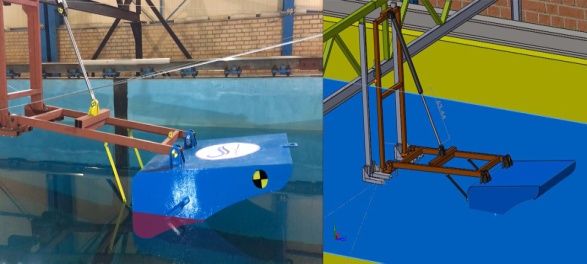
Fig. 1 Ro system and energy harvester from sea wave
Publications
1. R. Ahmadi, S. M. Pourfatemi, S. Ghaffari, Exergoeconomic optimization of hybrid system of GT, SOFC and MED implementing genetic algorithm, Desalination Volume 411, 1 June 2017, Pages 76–88
2. R. Ahmadi, T. Okawa, Influence of surface wettability on bubble behavior and void evolution in subcooled flow boiling, International Journal of Thermal Sciences 97, pp. 114-125, 2015
3. R. Ahmadi, T. Okawa, Recognition of Net Vapor Generation in Subcooled Flow Boiling, I Journal of Multidisciplinary Engineering Science and Technology (ISSN: 3159-0040), Vol. 2, Issue 3, pp. 465-469 (2015)
4. R. Ahmadi, T. Ueno, T. Okawa, Visualization study on the mechanisms of net vapor generation in water subcooled flow boiling under moderate pressure conditions International Journal of Heat and Mass Transfer, Vol. 70, pp. 137-151 (2014).
5. R. Ahmadi, T. Ueno, T. Okawa, Experimental identification of the phenomenon triggering the net vapor generation in upward subcooled flow boiling of water at low pressure, International Journal of Heat and Mass Transfer, Vol. 55, No. 21-22, pp. 6067-6076 (2012).
Seyed Jamaleddin Shahtaheri
Tehran University of Medical Sciences, Iran
Title: Optimization of solid phase microextraction procedure followed by GC-ECD for pesticides butachlor and chlorpyrifos

Biography:
Seyed Jamaleddin Shahtaheri has completed his PhD at the age of 38 years from Surrey University, Guildford, Surrey, England in 1996. He is an academic member of Department of Occupational Health Engineering, Tehran University of Medical Sciences, Iran, acting as the Dean Research Deputy at Institute for Environmental Research the same University too. Shahtaheri is the Persistent Organic Pollutant Review Committee (POPRC) Member under the Stockholm Convention, UNEP, UN. He has published more than 150 papers in reputed journals and has been serving as an editorial board member of 7 national and International Journals.
Abstract:
In this study, headspace solid phase microextraction (HS-SPME) followed by gas chromatography using electron capture detection system (GC-ECD) were developed for the determination of chloraacetanilide (butachlor) and chlorpyrifos present in biological samples. Different parameters affecting the extraction procedure were optimized including extraction time (30 minutes), extraction temperature (80°C), sample volume (3 mL), sample pH (2), added NaCl (0.3 gram) and sample stirring rate (400 rpm). Different concentrations of 1-100 ng/ml were applied for butachlor and a linear calibration curve was obtained. Furthermore, a similar linearity was obtained for chlorpyrifos, using a concentration range of 1-250 ng/ml. The limit of detection (LOD) obtained for butachlor and chlorpyrifos were 0.088 and 0.53 ng/ml respectively. The optimized methods for both compounds were validated using two concentrations of 25 and 50 ng/ml in spiked urine samples. Obtained recoveries of spiked urine samples were 83.06-99.8% with RSD of lower than 11%. Optimized technique was simple, inexpensive, solvent free and fast in comparison with other conventional methods and had compatibility with the chromatographic analytical system. This method offers low detection limits to analyze pesticides in urine samples that are very important in the exposure monitoring in occupational health.
James O. OJo
Federal Uiniversity of Technology, Nigeria
Title: Extractive separation of vanadium (v) and molybdenum (VI) from simulated 6M HCl solution with trioctyl phosphine oxide (TOPO)

Biography:
James O Ojo is skilled in the area of extractive metallurgy with strong passion for vanadium and molybdenum. His impressive output emanated from the many years of research and teaching on the aqueous chemistry of these metals, thus the versatility in the utilization of various extractants, including trioctyl phosphine oxide, and many other ligands for the extractive separation of these uncommonly associated metals. His ultimate interest, as well as success, is in the devising of simple extractive separation routes for co-polymerizing metals (from admixtures) such as vanadium and molybdenum, with vast applications in hydrometallurgy, recycling and routine analysis. This approach will eventually impart finesse to the metallurgical and recycling processes of several versatile metals.
Abstract:
The extractive separation of vanadium (V) and molybdenum (VI) from 6.0 mol dm-3 HCI in the presence of 0.1 mol dm-3 KCI as a salting -out agent using trioctyl phosphine oxide (TOPO) IN n-heptane has been investigated. Equilibrations were performed at optimal contact time of 5 min. The V (V) and Mo (VI) ions were determined spectrophotometrically by the phosphotungstate and thiocyanate methods respectively. From their individual synthetic solutions, the effect of increasing concentration of TOPO in the range 0.013-0.065moldm-3 to enhance a selective extraction, proved inefficient. This is because both ions were substantially extracted with percentage V(V) extraction (E%) optimized at 90.0% while Mo(VI) was 95.0%, with both observed at a concentration of 0.52 mol dm-3 TOPO. This was reflected in the low separation factor(![]() ) of the range 1.8-2.1. For the synthetic simulated mixture of the metals in 6.0 mol dm-3 HCl, parameters such as the effect of concentration of TOPO and organic to aqueous phase volume ratio (1:1- 4:1) on the percentage of the metal extracted or stripped was examined. The optimal separation of V(V) and Mo(VI) was achieved by selective stripping with percentage of V(V) and Mo(VI) stripped (separately) after two stages being 99.0 and 99.8% respectively. In this process we used 2.0 mol dm-3 H2SO4 and 14.5 mol dm-3 NH3 in that order, at organic to aqueous phase volume ratio (1:1 to 4:1). It decreased the percentage of the metals co-extracted and hence that available for stripping (E% of V(V) decreased from 90.0 to 75.0% and for Mo(VI) 96.0 to 57.1% for a single stage stripping. From the slope and spectral analysis , the stoichiometries of the composition of the organic phase were formulated as VO2Cl. 2TOPO and MoO2Cl2. TOPO.
) of the range 1.8-2.1. For the synthetic simulated mixture of the metals in 6.0 mol dm-3 HCl, parameters such as the effect of concentration of TOPO and organic to aqueous phase volume ratio (1:1- 4:1) on the percentage of the metal extracted or stripped was examined. The optimal separation of V(V) and Mo(VI) was achieved by selective stripping with percentage of V(V) and Mo(VI) stripped (separately) after two stages being 99.0 and 99.8% respectively. In this process we used 2.0 mol dm-3 H2SO4 and 14.5 mol dm-3 NH3 in that order, at organic to aqueous phase volume ratio (1:1 to 4:1). It decreased the percentage of the metals co-extracted and hence that available for stripping (E% of V(V) decreased from 90.0 to 75.0% and for Mo(VI) 96.0 to 57.1% for a single stage stripping. From the slope and spectral analysis , the stoichiometries of the composition of the organic phase were formulated as VO2Cl. 2TOPO and MoO2Cl2. TOPO.

Biography:
Maryam Sayah has completed her master's degree in 2016 from the Department of chemistry, North Tehran Branch, Islamic Azad University, Tehran, Iran. Her research project under the supervision of Dr Vahid Kiarostami was on the Development of a low density Solvent Based Solvent-Terminated Dispersive Liquid Liquid Microextraction (ST-DLLME) for the Extraction of acrylamide in water sample using central composite design (CCD). She is interested in chromatography (GC and HPLC), microextraction and chemometric methods.
Abstract:
Statement of the Problem: Polyacrylamide can be applied with multiple purposes in some industries such flocculants for clarification of potable water and treatment of municipal and industrial effluents and also used as grouting agents in the construction of drinking water reservoirs and wells, soil conditioning agents. Residual levels of unreacted acrylamide monomer in polyacrylamide can easily transfer to environmental and drinking water due to its water solubility and consequently causes, the most important source of water contamination. Acrylamide has been stated as carcinogenic to humans (Group 2A) with a lifetime cancer of 10-5 in 0.5 µg L-1 in drinking water. Methodology & Theoretical Orientation: A reliable, fast and safe method with easy operation for determination of acrylamide in water samples using a low density solvent based solvent-terminated dispersive liquid liquid microextraction (ST-DLLME) combined with GC-FID as a low cost detector which is available for most research laboratories has been developed and studied. Octanone and methanol were selected as the best extraction and dispersive solvents, respectively, using one factor at a time method. A central composite design (CCD) as a response surface methodology was used for multivariate optimization of the influences of other five factors (Volumes of extraction and dispersive solvents, PH, salt addition and extraction time) on the extraction efficiency. Under optimized condition (extraction solvent and its volume: Octanone, 175 µl; dispersive solvent and its volume: 683 µl; PH: 6.5; salt addition: 2.5% and extraction time: 4 min), the linear range was 0.3-550 ng mlˉ¹ with detection (S/N=3) and determination (S/N=10) limits of 0.1 and 0.3 ng mlˉ¹, respectively. The percentage recoveries of acrylamide from drinking and well water at spiking level of 0.5, 1 and 10 ng mlˉ¹ were obtained in the range of 90.8-94.1%. Compared with the previously reported methods, the proposed ST-DLLME method indicates adequately figures of merit.
Image
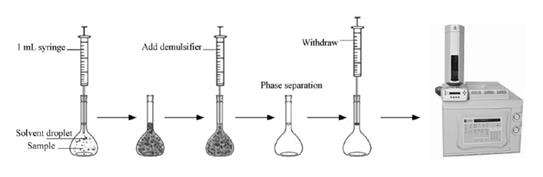
Figure 1: The graphical abstract of the proposed ST-DLLME method coupled GC-FID
Publications
1.Chen L, Haizhu L, Ping Y, Jinun Z, Xi C (2009) Determination of Acrylamide in Foods by Solid Phase Microextraction-Gas Chromatography. Food Science and Biotechnology 18(4):895-899.
2.Sobhi HR, Ghambarian M, Behbahani M, Esrafili A (2017) Application of modified hollow fiber liquid phase microextraction in conjunction with chromatography-electron capture detection for quantification of acrylamide in waste water samples at ultra-trace levels. J Chromatogr A 1487: 30-35.
3.Alpmann A, Morlock G (2008) Rapid and sensitive determination of acrylamide in drinking water by planar chromatography and fluorescence detection after derivatization with dansulfinic acid. Journal of separation science 31(1): 71-77.
4.Cavalli S, Polesello S, Saccani G (2004) Determination of acrylamide in drinking water by large-volume direct injection and ion-exclusion chromatography–mass spectrometry. Journal of Chromatography A 1039(1): 155-159.
Adolfo Iulianelli
Institute on Membrane Technology of the Italian National Research Council, Italy
Title: Membrane technology for H2 separation

Biography:
Adolfo Iulianelli has MSc Degree in Chemical Engineering in 2002 at University of Calabria (Italy), obtained his PhD in Chemical and Materials Engineering in 2006 at the same university. Nowadays, he is working at the Institute on Membrane Technology of the National Research Council of Italy (CNR-ITM). He is author and co-author of more than 120 papers, editorials and conference papers, one patent, two books and more than 20 book chapters. Furthermore, he is Reviewer of 30 international ICI journals, Invited/Keynote Speaker in more than 10 international conferences, training school, etc., Subject Editor of the Scientific World Journal, Guest Editor for the International Journal of Hydrogen Energy, Associate Editor of International Journal of Membrane Science and Technology and Editorial Member of other scientific journals. His research interests are Membrane reactors, Membrane technology in gas separation, Hydrogen production and reforming reactions, CO2 capture, etc. He has been involved in various Italian (5) and European (4) projects. His h-index is 24.
Abstract:
Nowadays, gas separation technologies play an important role at industrial level in a large number of chemical applications. Among them, it is worth of noting the pressure swing adsorption, temperature swing adsorption, liquid absorption and stripping, cryogenic distillation and, as emerging technology, the membrane gas separation (MGS). In most of cases, they compete each other for the same application, whereas for high product purity requirements, a combination of them should be requested. Nevertheless, the recovery and desired purity requirements and the scale operation constitute the main variables for the selection of a separation process. The operational and economic benefits and drawbacks due to the utilization of MGS technology over other gas separation processes have been largely reviewed and a growing progress has been observed in this field. Moreover, in recent years, membrane engineering has driven to significant innovations in products and processes, making membrane technologies as a valid alternative to conventional operations. Meanwhile, the application of membrane technology in the viewpoint of the Process Intensification Strategy has made possible relevant benefits in terms of high energy saving, better raw material exploitation, lower waste generation and dramatic reduction of equipment size. Among a number of potential applications in chemical processes, such polymeric membranes are particularly used in H2/N2 separation (ammonia synthesis process) and hydrogen recovery in refineries. However, in the last years also inorganic membrane technology has received a great attention, particularly for H2 separation from industrial gaseous streams via Pd-based membranes. Here an application of thermal treated PEEK-WC and PLA membranes for separating H2 from other gases of interest is presented. Furthermore, a comparison with the utilization of composite Pd-based MGS technology is given, consequently pointing out the advantages and disadvantages of both applications.
Image

Figure 1. Permeation characteristics of PLA membranes over Robeson plot - H2/CO2 selectivity vs H2 permeability upper-bound.
Publications
- Iulianelli A, Alavi M, Bagnato G, Liguori S, Wilcox J, Rahimpour MR, Eslamlouyan R, Anzelmo B, Basile A (2016) Supported Pd-Au membrane reactor for hydrogen production: membrane preparation, characterization and testing. Molecules 21:581-594.
- Liguori S, Iulianelli A, Dalena F, Pinacci P, Drago F, Broglia M, Huang Y, Basile A (2014) Performance and long-term stability of Pd/PSS and Pd/Al2O3 for hydrogen separation. Membranes 4:143-162.
- Briceño K, Montané D, Garcia-Valls R, Iulianelli A, Basile A (2012) Fabrication variables affecting the structure and properties of supported carbon molecular sieve membranes for hydrogen separation. Journal of Membrane Science 415-416:288-297.
- Iulianelli A, Basile A, Li H, Van Den Brink RW (2011) Inorganic membranes for pre-combustion carbon dioxide (CO2) capture, in Advanced membrane science and technology for sustainable energy and environmental applications. Woodhead Publishing Series in Energy – Cornwall (UK), Ch. 7, pp 184-213.
5. Iulianelli A, Liguori S, Morrone P, Basile A (2012) Membrane and membrane reactor technologies for COx purification of gaseous streams. Nova Science Publishers, New York (USA).
